Axonics SNM Systems are MRI conditionally safe for:
- 1.5T and 3T full-body MRI scans
- 1.5T and 3T head coil MRI scans
- 1.5T and 3T upper and lower extremity MRI scans
- 1.5T and 3T full-body coil with open impedances
- 1.5T and 3T full-body with lead fragments
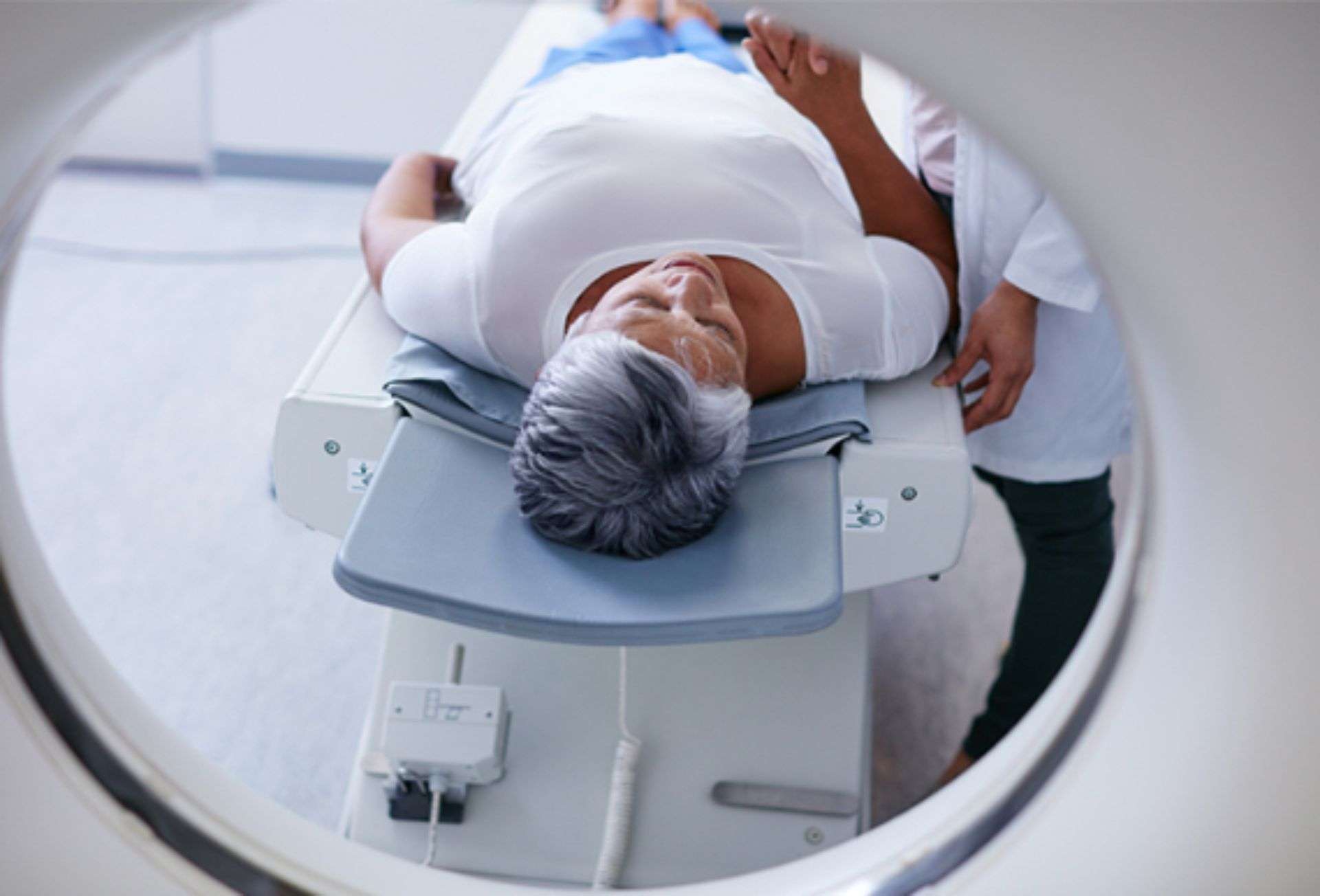
A patient implanted with the Axonics SNM Systems can undergo MRI examinations safely under the conditions outlined in the Axonics MRI Conditional Guidelines.
United States
Canada (English)
Canada (French)
Australia
Europe
- To receive a print copy of the MRI Guidelines, please email customer support at customersupport@axonics.com
 Technology
Technology
Axonics SNM Systems feature SmartMRITM Technology that simplifies the process for your patients to get an MRI. Using the Remote Control, the MR technician can easily confirm if a patient’s Axonics System is ready to undergo a full-body MRI. No office visit needed.

Axonics® Device MRI Safety by Component
The following components of Axonics SNM Systems can undergo MRI examinations safely under the conditions outlined in the Axonics MRI Conditional Guidelines:
- Implantable Neurostimulator (Model 1101, Model 4101, Model 5101)
- Tined Lead
The following components of the Axonics Systems are not safe to undergo MRI examinations and should not be brought into the magnet room:
- External Trial System (External Trial Stimulator, percutaneous leads, and cables)
- Patient Remote Control
- Charging System
- Clinician Programmer
Know the Conditional Guidelines
Prior to MRI examination, please read the Axonics MRI Conditional Guidelines carefully to learn about the conditions, potential risks, precautions, and instructions on using MRI with the Axonics Systems. MRI scans performed outside the guidelines may result in unwanted interaction of MRI fields with implanted devices, potentially injuring the patient and damaging the implanted device.

References:
1. Pezzella A, McCrery R, Lane F, et al. Two-year outcomes of the ARTISAN-SNM study for the treatment of urinary urgency incontinence using the Axonics rechargeable sacral neuromodulation system [published online ahead of print, 2021 Jan 28]. Neurourol Urodyn. 2021;10.1002/nau.24615. doi:10.1002/nau.24615
Questions?
We are here to help answer any questions you may have about Axonics Therapy.

