
Bulkamid® is Now a Part of the Axonics® Product Family
Axonics Doubles Down on its Commitment to Partner with Physicians to Treat Incontinence and Help Improve Quality of Life

Dear Valued Partners in Urology,
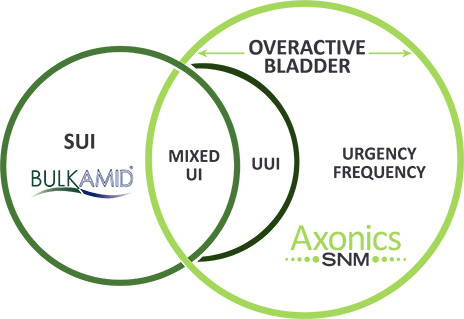
At Axonics, our mission and commitment are to provide solutions to improve the quality of lives of people with bladder and bowel dysfunction. To that end, we are pleased to share that we have acquired Bulkamid®, a unique, efficacious and durable bulking agent for the treatment of stress urinary incontinence (SUI) in women.
In late 2019, we introduced our innovative Sacral Neuromodulation (SNM) system to provide a long-lived implantable solution for people suffering from overactive bladder, urinary retention and fecal incontinence. Now, Bulkamid expands our ability to partner with Urologists and Urogynecologists to treat another underserved patient population, women suffering with SUI. The addition of Bulkamid to our product portfolio underscores Axonics' dedication to helping more of your patients.
Axonics® Now Offers Life-Changing Solutions for Patients with Overactive Bladder and SUI
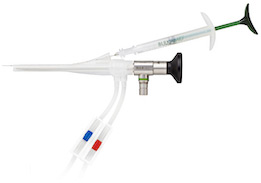
Bulkamid is an Innovative Bulking Agent to Treat Stress Incontinence
FDA approved in January 2020, Bulkamid is a novel, non-particulate hydrogel bulking agent that is injected into the urethral wall to help restore the natural closing pressure of the urethra. This innovative bulking agent:
- Addresses certain shortcomings of particulate-based bulking agents while offering an alternative to patients who want to avoid sling surgery and instead prefer a minimally invasive solution.
- Can be delivered in the office or an outpatient facility procedure.
- Is clinically proven to maintain durability and efficacy for out to seven years, providing women with safe and long-lasting relief of their SUI symptoms.1-3,5
- Was evaluated in a randomized controlled trial, with 92% of Bulkamid patients identifying as cured or improved at 12 months.4
- Employs a proprietary, easy-to-use injection system that allows precise injection of the non-particulate homogenous hydrogel.
Interested in using Bulkamid in your practice? Please provide your information through the link at the bottom to be contacted by an Axonics representative as we expand access to Bulkamid over the next few months.

Experience the Difference - Try the Axonics System for Sacral Neuromodulation
Axonics is dedicated to advancing its long-lived implantable Sacral Neuromodulation system for the treatment of OAB, UR and FI. Since FDA approval in November 2019, the system has been rapidly adopted with physicians and patients experiencing the Axonics difference and numerous advantages:
- Designed to offer the best patient and physician experience based on:
- 15+ years of battery life
- Broad full-body MRI conditions
- An intuitive and easy-to-use patient remote control
- Once-a-month recharging that takes an hour*
- Clinically proven therapy, with long-term prospective data demonstrating sustained success rates6:
- 93% of implanted patients had ≥50% reduction in UUI symptoms at 2-years
- 94% of patients were satisfied with their therapy at 2-years
We are excited to add another important therapy to our product portfolio and look forward to expanding our partnership with you.
Interested in learning more about the Axonics System or Bulkamid?
Click here to receive follow up from one of our representatives.
www.axonics.com
References:
- Pai A, Al-Singary W. Durability, safety and efficacy of polyacrylamide hydrogel (Bulkamid®) in the management of stress and mixed urinary incontinence: three year follow up outcomes. Cent European J Urol. 2015; 68(4): 428-433.
- Andrews et al. Bulkamid® - does the volume injected affect outcome? IUGA 2015 Poster.
- Lobodasch K et al. Seven-year efficacy and safety outcomes of Bulkamid for the treatment of stress urinary incontinence. Journal Neurourology and Urodynamics. 2021;40:502-508
- Freitas et al. Tension free vaginal tape vs polyacrylamide hydrogel injection for primary stress urinary incontinence: a randomized controlled trial. J Urol. Vol 203, 372 - 378, 2020.
- Mohr S, Marthaler C, Imboden S, Monga A, Mueller MD, Kuhn A. Bulkamid (PAHG) in mixed urinary incontinence: What is the outcome? Int Urogynecol J. 2017
- Pezzella A, McCrery R, Lane F, et al. Two-year outcomes of the ARTISAN-SNM study for the treatment of urinary urgency incontinence using the Axonics rechargeable sacral neuromodulation system [in press]. Neurourol Urodyn. 2021
* Under typical therapy settings
Important Safety Information:
Indications: Axonics SNM Therapy for urinary control is indicated for the treatment of urinary retention and the symptoms of overactive bladder, including urinary urge incontinence and significant symptoms of urgency-frequency alone or in combination, in patients who have failed or could not tolerate more conservative treatments. Axonics SNM Therapy for bowel control is indicated for the treatment of chronic fecal incontinence in patients who have failed or are not candidates for more conservative treatments.
Contraindications: The Axonics SNM System is contraindicated for patients who have not demonstrated an appropriate response to test stimulation; or Patients who are unable to operate the Axonics SNM System.
Warnings: Axonics SNM Therapy is not intended for patients with urinary mechanical obstructions. Diathermy cannot be performed on patients implanted with the Axonics SNM System. The Axonics SNM System is a MRI Conditional system. The following procedures may adversely affect the patient or the Axonics SNM System including: Lithotripsy, Monopolar electro surgery, Microwave and Radiofrequency (RF) ablation, Radiation therapy over the Neurostimulator, and Ultrasound or scanning equipment. Electromagnetic interference can interfere with the function of the Axonics SNM System. Walkthrough metal detectors, security archways, hand-held security wands should not affect the stimulator. Full-body security scanners are considered safe in patients that have the stimulator. Patients should minimize their exposure by not lingering in the immediate area of security systems. The Neurostimulator, Remote Control and Charger contain batteries with chemicals that can cause bodily harm, including severe burns, if exposed to your body. Do not rupture or pierce the devices or use the device that appears damaged or has visible internal components. If swelling or redness occurs near the Charger attachment site, discontinue to the use of Charger and consult your doctor before using the Charger again.
Precautions: The safety and effectiveness of the therapy has not been established for use in pregnant women, the unborn fetus, and during delivery, for pediatric patients (under the age of 18 years for FI and under the age of 16 years for OAB and UR), for patients with neurological disease origins, such as multiple sclerosis or diabetes, or for bilateral stimulation.
Caution: Federal law (USA) restricts this device to sale by, or on the order of, a physician.
© 2021 Axonics, Inc. All rights reserved.
26 Technology Drive. Irvine, CA 92618
Toll-Free: 1-877-9-AXONICS
Tel: +1-949-396-6320