Indications: The Axonics R20 System for urinary control is indicated for the treatment of the symptoms of overactive bladder, including urinary urge incontinence and significant symptoms of urgency-frequency alone or in combination, in patients who have failed or could not tolerate more conservative treatments. For a complete listing of indications, contraindications, warnings and precautions, go to www.axonics.com/isi

Sacral Neuromodulation System
The Axonics R20® System provides a best-in-class patient and physician experience.
Offer your patients the confidence of a long-lasting therapy.
- Battery that only needs recharging every 6 to 10 months*
- Designed to last 20+ years
- Miniaturised device, only 5 cc in volume
- Pocket-sized Patient Remote Control that doesn’t require recharging
- Expanded MRI access
- Dual program capability

Recharge-Free Patient Remote Control
- Pocket-sized and easy-to-use
- One-handed operation with no need for an extra communicator
- Indicates when it is time to recharge the R20 device
- Allows increase or decrease in stimulation
- Allows MR technician to check system readiness before full-body MRI
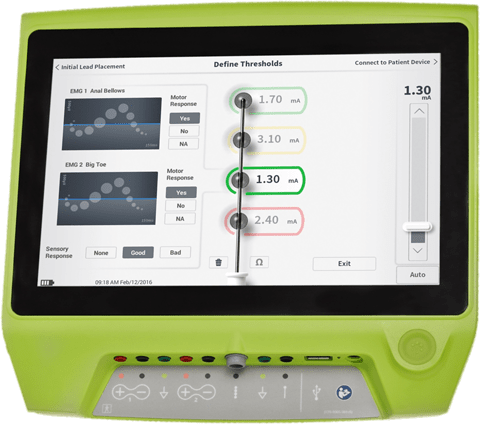
Fuss-Free Patient and Provider Experience
Programming the Axonics R20 is informed by our proprietary Smart Programming algorithm, including:
- Personalised therapy for every patient, with programming recommendations based on their lead placement.
- Accurate prediction of effective programming at implant, with minimal need for reprogramming1
- Minimising the need for patients to adjust their program1
In the ARTISTRY Registry study,

of patients were on one of their top two recommended programs after one year.1
Patented Tined Lead Design
- Patented helical tine design to mitigate lead migration
- Visible and radiopaque markers for lead implantation


Charging System
- Wireless charging system allows for patient mobility
- Charges wirelessly through the skin
- Only needs recharging every 6 to 10 months*

MRI conditionally safe for:
- 1.5T and 3T Full-Body Coil – expanded conditions

- 1.5T and 3T Head Coil
- 1.5T and 3T Upper and Lower Extremity Coils

Axonics SNM is Clinically Proven to Deliver Lasting Results
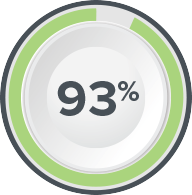
of patients had a ≥50% reduction in UUI symptoms at 2 years2
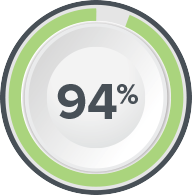
of patients were satisfied with their therapy2

No serious device-related adverse events2
References:
*Recharge interval depends on therapy settings
1. ARTISTRY Registry data on file
2. Pezzella A, McCrery R, Lane F, et al. Two-year outcomes of the ARTISAN-SNM study for the treatment of urinary urgency incontinence using the Axonics rechargeable sacral neuromodulation system. Neurourol Urodyn. 2021
Questions?
We are here to help answer any questions you may have about Axonics Therapy.
